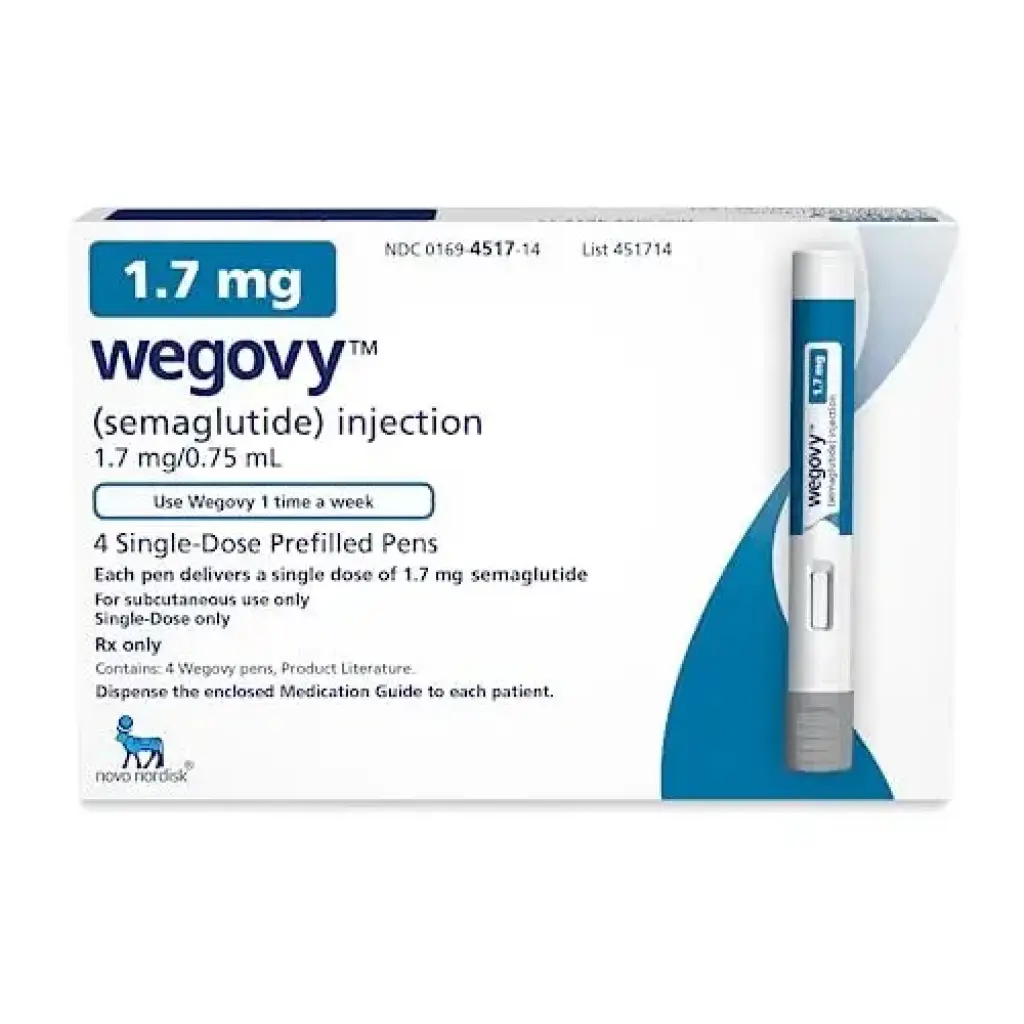


CLASSIFICATION: GLP-1 RECEPTOR AGONIST
ACTIVE SUBSTANCE: SEMAGLUTIDE
FORM: PRE-FILLED PEN (1.75 MG/PEN)
ACTIVE HALF-LIFE: ~7 DAYS
DOSAGE: 0.25 - 0.5 MG/WEEK
ACNE: NO
WATER RETENTION: NO
HIGH BLOOD PRESSURE (HBP): NO
HEPATOTOXICITY: NO
AROMATIZATION: NO
MANUFACTURER: NOVO NORDISK
Wegovy 1.75 mg is a pre-filled subcutaneous pen developed by Novo Nordisk, designed to deliver a weekly dose of Semaglutide as part of a progressive titration protocol. This intermediate-strength pen bridges the transition between 1.0 mg and the final 2.4 mg maintenance dose. It is intended strictly for research use in studies related to obesity, metabolic health, and GLP-1 receptor agonist effects.
Semaglutide is a long-acting GLP-1 analog that promotes satiety, slows gastric emptying, and improves insulin sensitivity. Its mechanism of action targets both peripheral and central pathways that regulate hunger and energy balance. In published clinical trials, Semaglutide induced significant reductions in body weight, waist circumference, and HbA1c levels when administered weekly.
This 1.75 mg pen is used after titration through 0.25 mg, 0.5 mg, and 1.0 mg doses. It precedes the full 2.4 mg maintenance dose and allows researchers to assess tolerance and optimize therapeutic effects. It is injected once per week subcutaneously, typically in the abdomen, thigh, or upper arm.
Semaglutide research protocols often benefit from stacking with other metabolic and regenerative compounds for broader impact:
Semaglutide has a 7-day half-life, enabling consistent plasma levels with weekly injections. GI discomfort such as nausea or early satiety may occur as the dose increases, but most effects subside over time. Semaglutide does not aromatize or disrupt hormone levels and is non-hepatotoxic. Its profile is favorable in both short- and long-term preclinical weight loss research.
Wegovy 1.75 mg pens are manufactured by Novo Nordisk and handled under cold-chain conditions. Dragon Pharma Store ensures fast, tracked USA domestic shipping with guaranteed quality. Also available are earlier titration phase options like Wegovy 1 mg or the injectable research-grade Dragon Pharma Semaglutide in vial form.
Wegovy 1.75 mg is used in the pre-maintenance phase of Semaglutide weight loss protocols to assess tolerance and effectiveness before reaching 2.4 mg.
It is administered via subcutaneous injection once per week using a pre-filled Novo Nordisk pen. Typical sites include the abdomen or thigh.
Yes, Semaglutide is often stacked with peptides like AOD 9604, CJC-1295, or GHK-Cu for enhanced fat loss, recovery, or insulin sensitivity protocols.
Wegovy 1.75 mg is typically a transitional dose, though some protocols may extend it longer before moving to the full 2.4 mg maintenance dose.
Please log in to write WEGOVY 1.75 MG review.

CLASSIFICATION: HUMAN PEPTIDE HORMONE
ACTIVE SUBSTANCE: SEMAGLUTIDE
FORM: LYOPHILIZED POWDER (2 ML VIAL x 5 MG)
ACTIVE HALF-LIFE: APPROXIMATELY 7 DAYS
DOSAGE: 0.25–2.4 MG WEEKLY
ACNE: NOT REPORTED
WATER RETENTION: NOT REPORTED
HIGH BLOOD PRESSURE (HBP): NOT REPORTED
HEPATOTOXICITY: NO
AROMATIZATION: NO
MANUFACTURER: PEPTIDE HUBS
LABORATORY TESTED: VIEW LAB RESULTS

CLASSIFICATION: GLP-1 RECEPTOR AGONIST
ACTIVE SUBSTANCE: SEMAGLUTIDE
FORM: 2 ML VIAL x 5 MG (LYOPHILIZED POWDER)
ACTIVE HALF-LIFE: ~7 DAYS
DOSAGE: 0.25 - 0.5 MG/WEEK
ACNE: NO
WATER RETENTION: NO
HIGH BLOOD PRESSURE (HBP): NO
HEPATOTOXICITY: NO
AROMATIZATION: NO
MANUFACTURER: DRAGON PHARMA, EUROPE
LABORATORY TESTED: VIEW LAB RESULTS

CLASSIFICATION: GLP-1 RECEPTOR AGONIST
ACTIVE SUBSTANCE: SEMAGLUTIDE
FORM: 2 ML VIAL x 10 MG (LYOPHILIZED POWDER)
ACTIVE HALF-LIFE: ~ 7 DAYS
DOSAGE: 0.25-0.5 MG WEEKLY, TITRATE AS NEEDED
ACNE: NOT REPORTED
WATER RETENTION: NOT REPORTED
HIGH BLOOD PRESSURE (HBP): UNLIKELY
HEPATOTOXICITY: NO
AROMATIZATION: NO
MANUFACTURER: STEALTH LABS
LABORATORY TESTED: VIEW LAB RESULTS
SHIPPED WITHOUT LABEL!